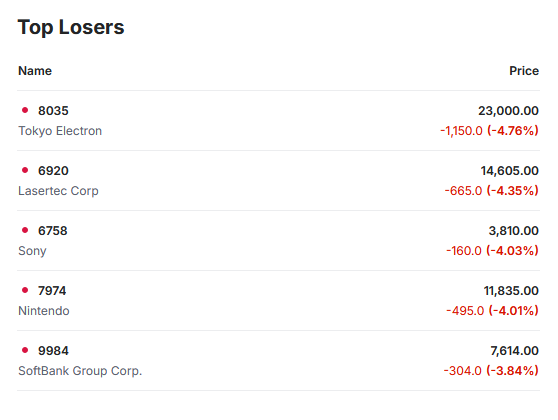
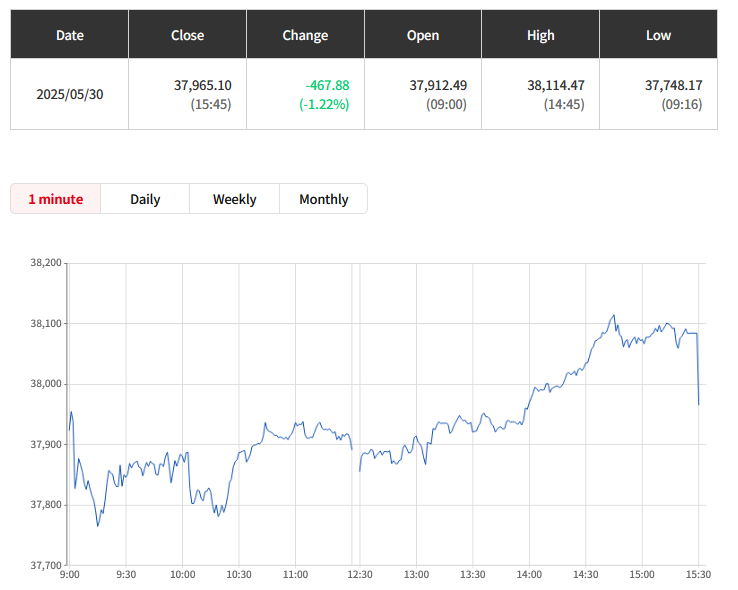
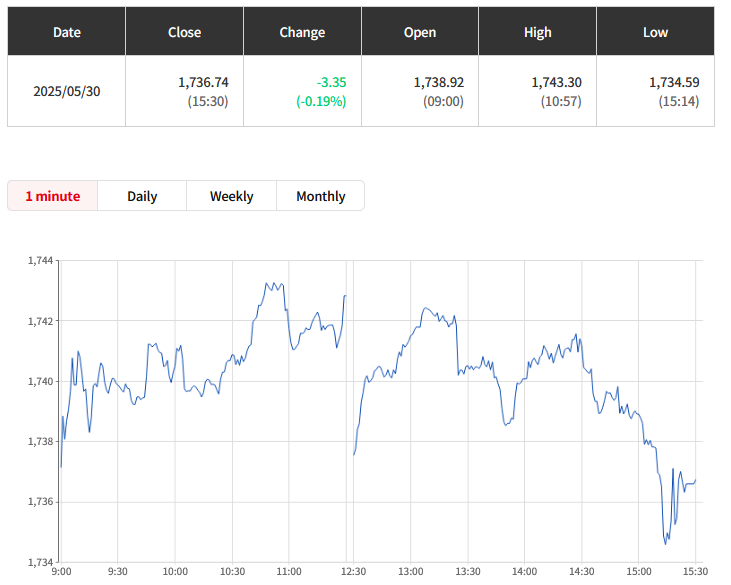
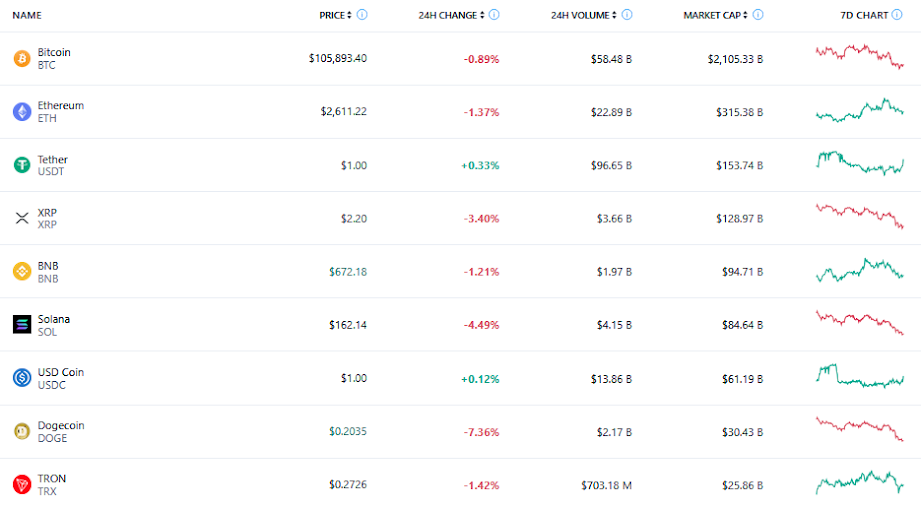
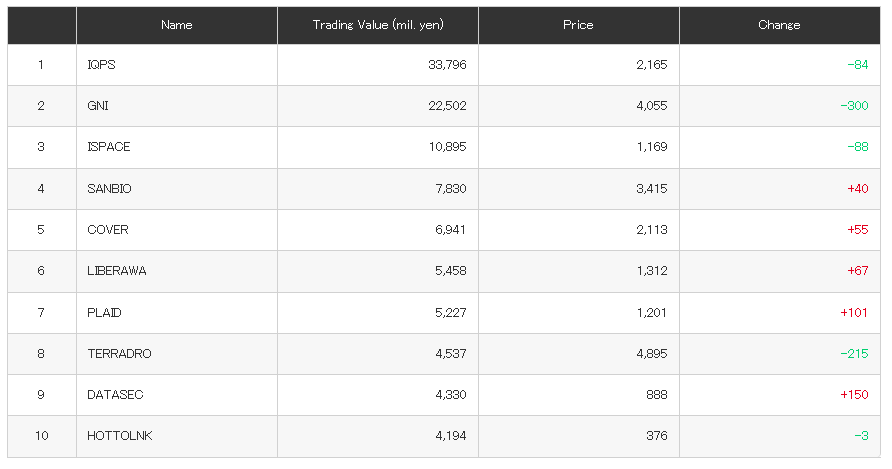
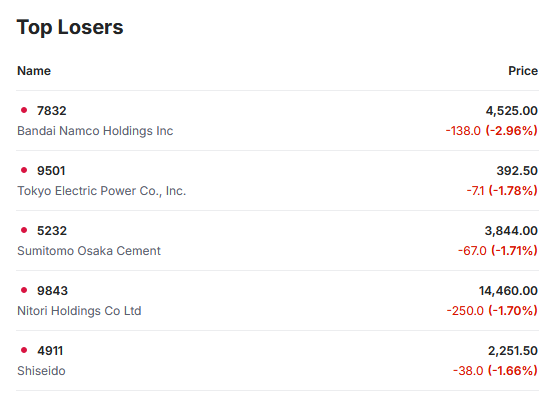
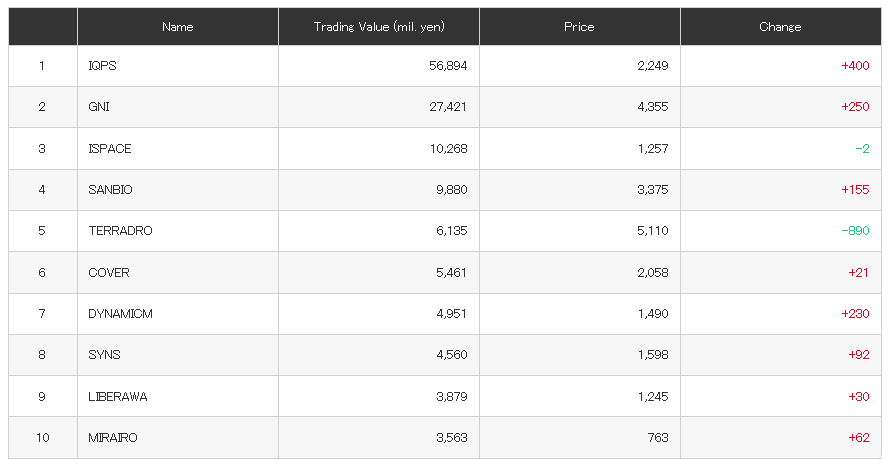
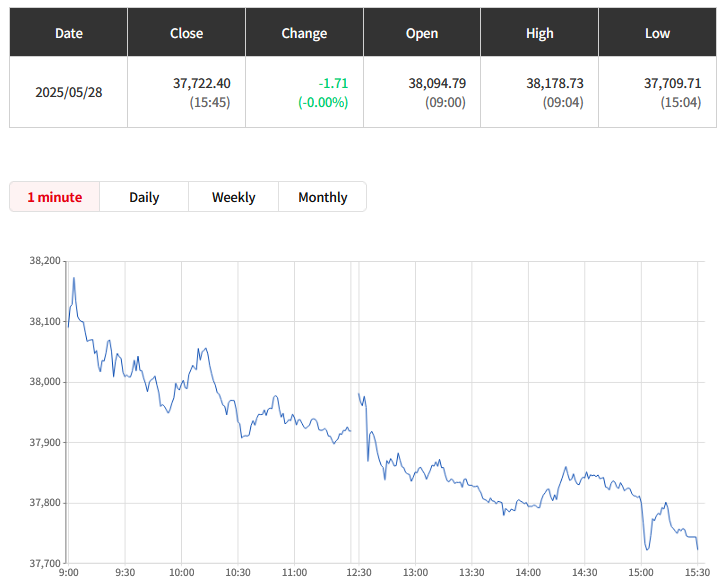
Otsuka achieves FDA Acceptance and Priority Review for new kidney drug Sibeprenlimab in the treatment of progressive, autoimmune, chronic kidney disease

The FDA target action date (PDUFA date) is set for November 28, 2025 If approved, sibeprenlimab would offer patients a convenient single-dose prefilled syringe for subcutaneous injection every four weeks intended for at home self-administration Sibeprenlimab has Breakthrough Therapy designation based on favorable results from the Phase 2 (ENVISION) trial (NCT04287985) 26 May 2025 Monday - Otsuka Pharmaceutical, Co. Ltd. (Otsuka) and Otsuka Pharmaceutical Development & Commercialization, Inc. (OPDC) announce the U.S. Food and Drug Administration (FDA) has accepted for review the Biologics License Application (BLA) for sibeprenlimab, an investigational monoclonal antibody that selectively inhibits the activity of APRIL ( A PR oliferation- I nducing L igand) in adults with immunoglobulin A nephropathy (IgAN). APRIL plays a key role in the pathogenesis of IgAN as explained by the 4-hit process, in which pathogenic galactose-deficient IgA (Gd-IgA1) is produced, lead...